Biostatistics for Innovative Clinical Trials in Würzburg
Symposium of the NCT WERA and DKFZ
In this symposium, renowned national and international experts will present new methodological aspects and developments in the field of early clinical trials in oncology, as well as discuss challenges in the planning and analysis of oncology trials. Furthermore, there will be room for direct scientific exchange between clinicians and methodologists on specific research questions in planning and conducting early clinical trials within the setting of dedicated workshops.
Presentations
Key notes (20 min. talks)
Professor Thomas Jaki, University of Regensburg, Regensburg, Germany
Dose finding with multiple questions
Professor Christina Yap, The Institute of Cancer Research, London, UK
The role of Patient-Reported Outcomes (PROs) in Early Phase and Adaptive Trials
Professor James Wason, Newcastle University, Newcastle upon Tyne, UK
Utilising predictive biomarkers for patient stratification in clinical trials
Professor Christophe Le Tourneau, Institute Curie, Paris, France
Current challenges for drug development in the era of precision medicine in oncology
Specific aspects and practical challenges in early clinical trials in oncology (10 min. talks)
Dr. Lucie Biard, MCU-PH chez Université Paris Cité, AP-HP Hôpital Saint Louis, Paris, France
Late onset toxicity in dose-finding clinical trials
Dr. Philip Pallmann, Centre for Trials Research, Cardiff University, Wales, UK
Tools supporting planning and analysis of dose escalation trials
Dr. Silvia Calderazzo, German Cancer Research Center (DKFZ), Heidelberg, Germany
Using external data to augment decision making in small populations
Dr. Dominic Edelmann, German Cancer Research Center (DKFZ), Heidelberg, Germany
Improving Single Arm Trials Using Patient Characteristics and External Data
Dr. Pavel Mozgunov, University of Cambridge, Cambridge, UK
Development and implementation of adaptive designs in early phase clinical trials
Agenda (preliminary) – Tuesday, July 1st
11.00 AM Beginning of the event/Introduction NCT WERA
11.15 AM - 12.40 AM Key note lectures
12.40 PM - 1.30 PM Specific aspects and practical challenges in early clinical trials in oncology
1.30 PM Lunch break
2.30 PM - 4.00 PM Clinicians meet Biostatisticians (working groups)
4.00 PM - 5.00 PM Plenary Session and Summary
5.00 PM End of the event
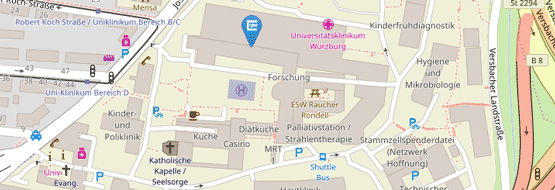
Location
University Hospital Würzburg
Department of Internal Medicine (ZIM)
Oberdürrbacher Str. 6
97080 Würzburg
Hörsaal ZIM 1
We look forward to welcoming you in person.
Click here to register
https://indico.dkfz.de/e/symposium_biostatistik_nctwera
Join via Zoom
https://ukw-de.zoom.us/j/92730857595?pwd=vj6kNgOVpgaGDCKSaxSjr9GCNGZwys.1
Organizer
University Hospital Würzburg
Host
NCT WERA