Host-microbial interactions (Gomez de Agüero Lab)
At birth, mammalian barrier tissues get colonized by trillions of microorganisms, which collectively form the commensal microbiota. This is the major supplier of metabolites involved in essential processes for the host, such as energy stockage and training of the mammalian immunity.
Recently, we and others have defined a critical window early in life for the microbiota to shape the immune system. Our previous work revealed the pivotal role of the microbiota during pregnancy and lactation for the development of the intestinal immune system. Indeed, maternal microbial-derived metabolites reach the offspring during the gestation and the lactation and shape the intestinal immunity to prepare the newborn for the challenges of birth.
The focus of our lab is to understand the crosstalk of the microbiota and its products with host cellular networks. Specifically, we try to elucidate which and how specific molecules mediate the effects of the microbiota on a mechanistic level. Functionally, we focus on the role of microbial derived metabolites on physiology and development.
We use a sophisticated gnotobiotic research model based on an auxotrophic E. coli strain for exclusive gestational colonization with sterile offspring. Flow cytometry, histology, single cell RNA sequencing, and metabolic analysis of the perinatal skin allowed us to show that maternal microbiota shapes the development of the skin through the regulation of gene expression of epidermal stem cells. Thus, the differentiation of the keratinocytes to a more mature or specialized stage is impaired in the absence of maternal microbial signals. Unbiased metabolic analysis showed that circa 350 metabolites accumulate in the neonatal skin upon microbial exposure of the mothers. About 75% of them have a microbial origin, such as tryptophane, nicotinamide, essential amino acids, and vitamin B6 and derivates. Using our murine model and cyst-based organoid model, we have shown that carriers and receptors of these metabolites are enhanced in the epidermal stem cells. Consequently, neonatal permeability barrier and its regeneration is enhanced by gestational colonization.
Current projects investigate the impact of maternal microbiota and its metabolites on promoting the proper development of the skin barrier in newborns to prevent infections, autoimmune diseases, and allergy.
Intestinal Bacteria Trigger Postoperative Complications
Previously, it was believed that a germ-free environment was the most critical factor in preventing postoperative infections. However, a recent study by Mercedes Gomez de Agüero's team from the Max Planck Research Group for Systems Immunology in Würzburg, Germany, in collaboration with the University Hospital of Bern, Switzerland, has revealed that the source of the danger is apparently entirely different: the patients' intestines.
Hospitals have traditionally sought to prevent infections after surgical procedures by maintaining a germ-free environment. It has long been established that concomitant infections during invasive procedures increase mortality rates. For this reason, extensive sterilization measures are implemented to eliminate microorganisms during surgery.
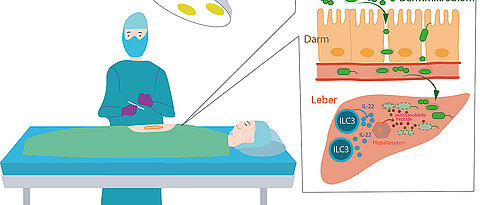
The research team's study, published in Cell Reports, shows that the causative agents of these infections have a microbial signature from the intestine. In nearly all patients, the pathogens were intestinal bacteria such as Enterococcus, Escherichia coli, and Clostridium. The research team analyzed the microorganisms that caused concomitant infections in almost 4,000 patients after major surgery.
The pathogens seem to breach the intestinal barrier postoperatively and spread throughout the body via the bloodstream. These infections frequently occur after surgeries on the liver, pancreas, and bile ducts, as well as during operations on the small and large intestine. Patients who have undergone major liver resection, which involves removing large parts of the liver, are especially vulnerable to such infections, which significantly impede the healing process.
Crucial immune cells are present in the liver
The researchers then traced the infection control mechanisms in healthy patients. The liver plays a vital role in this process. "We know that unique immune cells residing in the liver are responsible for controlling these spreading bacteria and the healing process after major surgery," says Mercedes Gomez de Agüero, a junior research group leader in the Max Planck Research Group for Systems Immunology. They are a group of lymphocytes known as innate lymphoid cells (ILCs), which are crucial players in the innate immune system.
If bacteria from the intestine enter the liver through the bloodstream, these ILCs are activated and release specific messenger substances, such as interleukin 22, a protein that can trigger and regulate immune responses. In this manner, they induce liver cells to produce antimicrobial substances. "Innate lymphoid cells residing in the liver thus control the systemic spread of intestinal bacteria and effectively combat concomitant infections after surgery," she adds.
How can patients be protected from infections caused by their gut bacteria?
"Boosting immunity, therefore, represents a sensible prophylactic and therapeutic alternative strategy to the usual antimicrobial therapies to prevent concomitant infections after surgery," suggests Bern-based physician Guido Beldi. This approach should be considered until the factors responsible for gut bacteria penetrating the body's interior after surgical intervention have been identified. The research team intends to investigate this issue next.
Original publication
ILC3s restrict the dissemination of intestinal bacteria to safeguard liver regeneration after surgery. Manuel O. Jakob, Daniel Spari, Daniel Sanchez-Taltavull, Lilian Salm, Bahtiyar Yilmaz, Remi Doucet Ladeveze, Catherine Mooser, David Pereyra, Ye Ouyang, Theresa Schmidt, Irene Mattiola, Patrick Starlinger, Deborah Stroka, Franziska Tschan, Daniel Candinas, Georg Gasteiger, Christoph S.N. Klose, Andreas Diefenbach, Mercedes Gomez de Agüero, Guido Beldi. Cell Reports, DOI: https://doi.org/10.1016/j.celrep.2023.112269
https://pubmed.ncbi.nlm.nih.gov
Selected Articles
- ILC3s restrict the dissemination of intestinal bacteria to safeguard liver regeneration after surgery, Manuel O. Jakob, Daniel Spari, Daniel Sànchez Taltavull, Lilian Salm, Bahtiyar Yilmaz, Rémi Doucet Ladevèze, Catherine Mooser, David Pereyra, Ye Ouyang, Theresa Schmidt, Irene Mattiola, Patrick Starlinger, Deborah Stroka, Franziska Tschan, Daniel Candinas, Georg Gasteiger, Christoph S.N. Klose, Andreas Diefenbach, Mercedes Gomez de Agüero, Guido Beldi. Cell Reports. 2023. doi: 10.1016/j.celrep.2023.112269
- Resident macrophages acquire innate immune memory in staphylococcal skin infection. Feuerstein R, Forde AJ, Lohrmann F, Kolter J, Ramirez NJ, Zimmermann J, Gomez de Agüero M, Henneke P. Elife. 2020 Jul 8;9:e55602. doi: 10.7554/eLife.55602.
- Uncoupling of invasive bacterial mucosal immunogenicity from pathogenicity Pfister SP, Schären OP, Beldi L, Printz A, Notter MD, Mukherjee M, Li H, Limenitakis JP, Werren JP, Tandon D, Cuenca M, Hagemann S, Uster SS, Terrazos MA, Gomez de Agüero M, Schürch CM, Coelho FM, Curtiss R 3rd, Slack E, Balmer ML, Hapfelmeier S.. Nat Commun. 2020 Apr 24;11(1):1978. doi: 10.1038/s41467-020-15891-9.
- Microbiota-induced tissue signals regulate ILC3-mediated antigen presentation. Lehmann FM, von Burg N, Ivanek R, Teufel C, Horvath E, Peter A, Turchinovich G, Staehli D, Eichlisberger T, Gomez de Agüero M, Coto-Llerena M, Prchal-Murphy M, Sexl V, Bentires-Alj M, Mueller C, Finke D. Nat Commun. 2020 Apr 14;11(1):1794. doi: 10.1038/s41467-020-15612-2.
- Neuronal programming by microbiota regulates intestinal physiology. Obata Y, Castaño Á, Boeing S, Bon-Frauches AC, Fung C, Fallesen T, de Agüero MG, Yilmaz B, Lopes R, Huseynova A, Horswell S, Maradana MR, Boesmans W, Vanden Berghe P, Murray AJ, Stockinger B, Macpherson AJ, Pachnis V. Nature. 2020 Feb;578(7794):284-289. doi: 10.1038/s41586-020-1975-8.
- NLRP6 Deficiency in CD4 T Cells Decreases T Cell Survival Associated with Increased Cell Death.Radulovic K, Ayata CK, Mak'Anyengo R, Lechner K, Wuggenig P, Kaya B, Hruz P, Gomez de Agüero M, Broz P, Weigmann B, Niess JH. J Immunol. 2019 Jul 15;203(2):544-556. doi: 10.4049/jimmunol.1800938.
- Antibodies Set Boundaries Limiting Microbial Metabolite Penetration and the Resultant Mammalian Host Response. Uchimura Y, Fuhrer T, Li H, Lawson MA, Zimmermann M, Yilmaz B, Zindel J, Ronchi F, Sorribas M, Hapfelmeier S, Ganal-Vonarburg SC, Gomez de Agüero M, McCoy KD, Sauer U, Macpherson AJ. Immunity. 2018 Sep 18;49(3):545-559.e5. doi: 10.1016/j.immuni.2018.08.004.
- Functional Gut Microbiota Remodeling Contributes to the Caloric Restriction-Induced Metabolic Improvements. Fabbiano S, Suárez-Zamorano N, Chevalier C, Lazarević V, Kieser S, Rigo D, Leo S, Veyrat-Durebex C, Gaïa N, Maresca M, Merkler D, Gomez de Agüero M, Macpherson A, Schrenzel J, Trajkovski M. Cell Metab. 2018 Dec 4;28(6):907-921.e7. doi: 10.1016/j.cmet.2018.08.005.
- Intestinal dendritic cell licensing through Toll-like receptor 4 is required for oral tolerance in allergic contact dermatitis. Hacini-Rachinel F, Gomez de Agüero M, Kanjarawi R, Moro-Sibilot L, Le Luduec JB, Macari C, Boschetti G, Bardel E, Langella P, Dubois B, Kaiserlian D. J Allergy Clin Immunol. 2018 Jan;141(1):163-170. doi: 10.1016/j.jaci.2017.02.022. Epub 2017 Mar 22.
- The maternal microbiota drives early postnatal innate immune development.Gomez de Agüero M, Ganal-Vonarburg SC, Fuhrer T, Rupp S, Uchimura Y, Li H, Steinert A, Heikenwalder M, Hapfelmeier S, Sauer U, McCoy KD, Macpherson AJ. Science. 2016 Mar 18;351(6279):1296-302. doi: 10.1126/science.aad2571.
- Langerhans cells protect from allergic contact dermatitis in mice by tolerizing CD8(+) T cells and activating Foxp3(+) regulatory T cells. Gomez de Agüero M, Vocanson M, Hacini-Rachinel F, Taillardet M, Sparwasser T, Kissenpfennig A, Malissen B, Kaiserlian D, Dubois B. J Clin Invest. 2012 May;122(5):1700-11. doi: 10.1172/JCI59725.
Selected Review Articles
- Standardization in host-microbiota interaction studies: challenges, gnotobiology as a tool, and perspective. Mooser C, Gomez de Agüero M, Ganal-Vonarburg SC. Curr Opin Microbiol. 2018 Aug;44:50-60. doi: 10.1016/j.mib.2018.07.007.
- The immunological functions of the Appendix: An example of redundancy? Girard-Madoux MJH, Gomez de Agüero M, Ganal-Vonarburg SC, Mooser C, Belz GT, Macpherson AJ, Vivier E. Semin Immunol. 2018 Apr;36:31-44. doi: 10.1016/j.smim.2018.02.005.
- How nutrition and the maternal microbiota shape the neonatal immune system. Macpherson AJ, de Agüero MG, Ganal-Vonarburg SC. Nat Rev Immunol. 2017 Aug;17(8):508-517. doi: 10.1038/nri.2017.58.
- Maternal microbiota and antibodies as advocates of neonatal health Ganal-Vonarburg SC, Fuhrer T, Gomez de Agüero M.. Gut Microbes. 2017 Sep 3;8(5):479-485. doi: 10.1080/19490976.2017.1299847. Erratum for: Addendum to: Gomez de Aguero M.*, , Ganal-Vonarburg S.C.*, , Fuhrer T., , Rupp S., , Uchimura Y., , Li H., , Steinert A., , Heikenwalder M., , Hapfelmeier S., , Sauer U., , McCoy K.D., , Macpherson A. J. (2016). The maternal microbiota drives early postnatal innate immune development. Science 351, 1296-1302. *Co-first authors. PMID: 28296565; PMCID: PMC5628635.
SNF PZ00P3_168012: Role of maternal microbiota and aryl hydrocarbon receptor signalling in establishing neonatal skin homeostasis



Özge Gokdag