Junior Research Groups
Kretzschmar Group

Adult Stem Cell Niches in Cancer
Riedel Group

Metastases, Metabolism and the Microenvironment
The MSNZ Würzburg currently supports four independent junior research groups. The main laboratories are located in the Institute for Virology and Immunobiology on the medical campus in Würzburg-Grombühl. The local Comprehensive Cancer Centre (CCC Mainfranken), the Max Planck Research Group for Systems Immunology, the Rudolf Virchow Centre and the Institute of Pathology are all nearby.
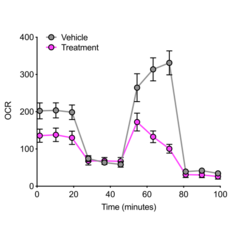
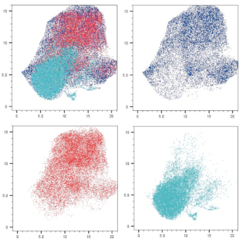
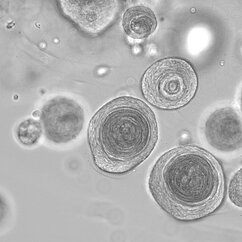
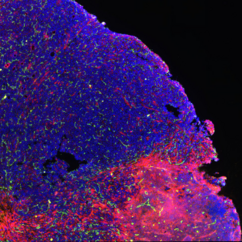
Our research is centered around the communication within the microenvironment in homeostasis and malignancies. Latest by the advent of single-cell sequencing technologies, it has become clear that tumors consist of complex tissue environments harboring tissue-resident and recruited cell populations. This does not only apply to the primary tumor, but also to its metastatic sites. Furthermore, this permissive microenvironment can support cancer progression and therapy resistance.
To understand the principles of gene expression regulation and their alterations in cancer, we also focus on nuclear paraspeckles. Components of paraspeckles regulate various RNA metabolic processes, are frequently upregulated in cancer and linked to genome stability.
Main research questions we try to answer:
-
What are the characteristics of the tumor cell survival niche and how can critical cell-cell interactions be targeted?
-
How do immuno- and chemotherapies impact the architecture of tumor cell clonality and their microenvironment?
-
In which way does the micro-environment contribute to metastatic colonization and which tumor-derived factors prime the metastatic niche?
-
What are the molecular processes underlying malignant transformation and do paraspeckles promote tumorigenesis?