New MSNZ publication - Rasche Group
07.09.2022The spatio-temporal evolution of multiple myeloma from baseline to relapse-refractory states
Recent longitudinal studies in Multiple Myeloma (MM) have identified a number of genetic aberrations, which are associated with increased clonal fitness and can lead to relapse. These aberrations frequently arise through branching pathways, especially in patients relapsing from deep responses. However, the origin of relapse subclones, as well as the extent of clonal diversity in treatment-resistant disease, remain largely unknown.
In this work, Leo and colleagues analyze longitudinal multi-region whole-exome sequencing data together with matched clinical annotations, including a total of 140 tumor samples from 24 MM patients. By tracking resistant clones in time and space they dissect the sophisticated spatiotemporal evolutionary pathways that are active in MM during treatment and demonstrate the power of combining functional imaging and tumor sequencing to delineate a more complete picture of subclonal evolution in MM.
Abstract
Deciphering Multiple Myeloma evolution in the whole bone marrow is key to inform curative strategies. Here, we perform spatial-longitudinal whole-exome sequencing, including 140 samples collected from 24 Multiple Myeloma patients during up to 14 years.
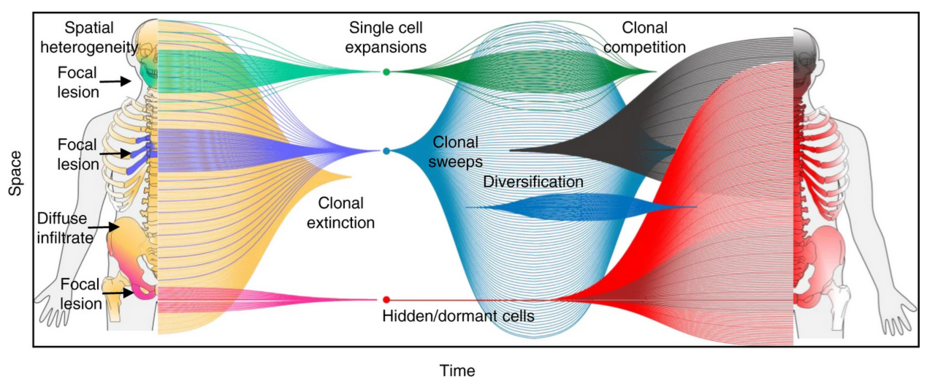
Applying imaging-guided sampling we observe three evolutionary patterns, including relapse driven by a single-cell expansion, competing/co-existing sub-clones, and unique sub-clones at distinct locations. While we do not find the unique relapse sub-clone in the baseline focal lesion(s), we show a close phylogenetic relationship between baseline focal lesions and relapse disease, highlighting focal lesions as hotspots of tumor evolution. In patients with ≥3 focal lesions on positron-emission-tomography at diagnosis, relapse is driven by multiple distinct sub-clones, whereas in other patients, a single-cell expansion is typically seen (p < 0.01).
Notably, we observe resistant sub-clones that can be hidden over years, suggesting that a prerequisite for curative therapies would be to overcome not only tumor heterogeneity but also dormancy.
©2022 The Authors; This is an open-access article, published in Nat Commun 13:4517.
Here you can find the full article in Nature Communications.