Research
Our research is focused on the cell biology of mesenchymal stromal cells (MSC) of different tissue origins. MSC populations have been described in various tissues. Based on their ability to give rise to different cell types (e.g. adipocytes, chondrocytes and osteoblasts) and their secretory potential they are of great interest in Regenerative Medicine. Our projects aim to decipher interactions of MSC with their environment, both in physiology and pathophysiology. Increasing the knowledge on such interactions as well as mechanisms of adaptation in course of degenerative diseases, will facilitate the development of new therapeutic approaches.
Currently, we are working on following topics:
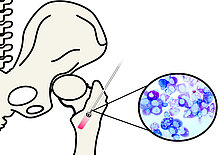
New therapeutic strategies for reconstruction of bone defects with optimized RIA-derived autologous grafts and bone substitute materials - RIABONE
Sebastian Häusner, Bianca Schlierf, Theresa Kreuzahler, Marietta Herrmann
Collaborators: Torsten Blunk (Würzburg), Aleksandra Jaukovic, Dusko Spasovski, Petar S. Milosavljević (Belgrad), OSARTIS GmbH
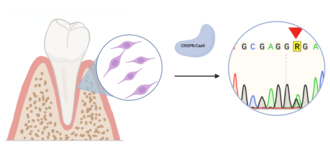
Consequences of gene defects in periodontal ligament MSCs
Katharina Marnet, Jana Schiffmaier, Theresa Kreuzahler, Drenka Trivanovic, Stephanie Graser (AG Hypophosphatasie Experimentell), Marietta Herrmann
Learn more...
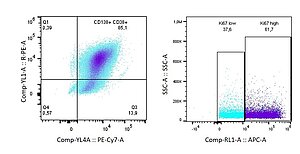
Bone marrow stromal cells in multiple myeloma
Jovana Ilic, Christoph Kölbl, Bianca Schlierf, Marietta Herrmann, Drenka Trivanovic
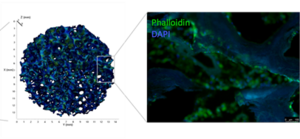
The extracellular matrix environment of bone marrow stromal cells
Ana Rita Pereira, Drenka Trivanovic, Bianca Schlierf, Theresa Kreuzahler, Marietta Herrmann
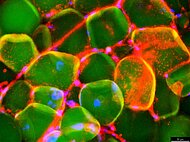
Identification and metabolic profiling of bone marrow adipose tissue progenitors
Annika Schiminski, Christoph Kölbl, Bianca Schlierf, Marietta Herrmann, Drenka Trivanovic