The human cognition-enhancing CORD7 mutation increases active zone number and synaptic release
01/12/2022The human cognition-enhancing CORD7 mutation increases active zone number and synaptic release
Mila M. Paul, Sven Dannhäuser, Lydia Morris, Achmed Mrestani, Martha Hübsch, Jennifer Gehring, Georgios N. Hatzopoulos, Martin Pauli, Genevieve M. Auger, Grit Bornschein, Nicole Scholz, Dmitrij Ljaschenko, Martin Müller, Markus Sauer, Hartmut Schmidt, Robert J. Kittel, Aaron DiAntonio, Ioannis Vakonakis, Manfred Heckmann and Tobias Langenhan
Brain, awac011, 2022
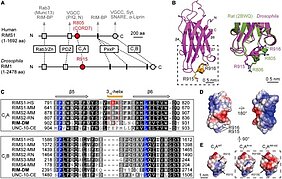
Humans carrying the CORD7 (cone-rod dystrophy 7) mutation possess increased verbal IQ and working memory. This autosomal dominant syndrome is caused by the single-amino acid R844H exchange (human numbering) located in the 310 helix of the C2A domain of RIMS1/RIM1 (Rab3-interacting molecule 1). RIM is an evolutionarily conserved multi-domain protein and essential component of presynaptic active zones, which is centrally involved in fast, Ca2+-triggered neurotransmitter release. How the CORD7 mutation affects synaptic function has remained unclear thus far. Here, we established Drosophila melanogaster as a disease model for clarifying the effects of the CORD7 mutation on RIM function and synaptic vesicle release.
To this end, using protein expression and X-ray crystallography, we solved the molecular structure of the Drosophila C2A domain at 1.92 Å resolution and by comparison to its mammalian homolog ascertained that the location of the CORD7 mutation is structurally conserved in fly RIM. Further, CRISPR/Cas9-assisted genomic engineering was employed for the generation of rim alleles encoding the R915H CORD7 exchange or R915E,R916E substitutions (fly numbering) to effect local charge reversal at the 310 helix. Through electrophysiological characterization by two-electrode voltage clamp and focal recordings we determined that the CORD7 mutation exerts a semi-dominant rather than a dominant effect on synaptic transmission resulting in faster, more efficient synaptic release and increased size of the readily releasable pool but decreased sensitivity for the fast calcium chelator BAPTA. In addition, the rim CORD7 allele increased the number of presynaptic active zones but left their nanoscopic organization unperturbed as revealed by super-resolution microscopy of the presynaptic scaffold protein Bruchpilot/ELKS/CAST.
We conclude that the CORD7 mutation leads to tighter release coupling, an increased readily releasable pool size and more release sites thereby promoting more efficient synaptic transmitter release. These results strongly suggest that similar mechanisms may underlie the CORD7 disease phenotype in patients and that enhanced synaptic transmission may contribute to their increased cognitive abilities.