Munc13-3 Is Required for the Developmental Localization of Ca2+ Channels to Active Zones and the Nanopositioning of Cav2.1 Near Release Sensors
01/01/2018Kusch V, Bornstein G, Loreth D, Bank J, Jordan J, Baur D, Watanabe M, Kulik A, Heckmann M, Eilers J, Schmidt H (2018)
Cell Reports 22, 1965–73
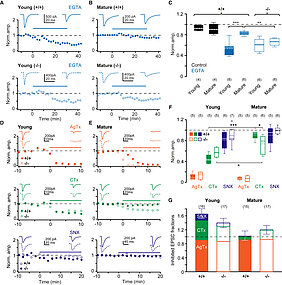
Spatial relationships between Cav channels and release sensors at active zones (AZs) are a major determinant of synaptic fidelity. They are regulated developmentally, but the underlying molecular mechanisms are largely unclear. Here, we show that Munc13-3 regulates the density of Cav2.1 and Cav2.2 channels, alters the localization of Cav2.1, and is required for the development of tight, nanodomain coupling at parallel-fiber AZs. We combined EGTA application and Ca2+-channel pharmacology in electrophysiological and two-photon Ca2+ imaging experiments with quantitative freeze-fracture immunoelectron microscopy and mathematical modeling. We found that a normally occurring developmental shift from release being dominated by Ca2+ influx through Cav2.1 and Cav2.2 channels with domain overlap and loose coupling (microdomains) to a nanodomain Cav2.1 to sensor coupling is impaired in Munc13-3-deficient synapses. Thus, at AZs lacking Munc13-3, release remained triggered by Cav2.1 and Cav2.2 microdomains, suggesting a critical role of Munc13-3 in the formation of release sites with calcium channel nanodomains.