Agonists binding nicotinic receptors elicit specific channel opening patterns at αγ or αδ sites
01/01/2014Stock P, Ljaschenko D, Heckmann M, Dudel J (2014)
J Physiol 592:2501-2517
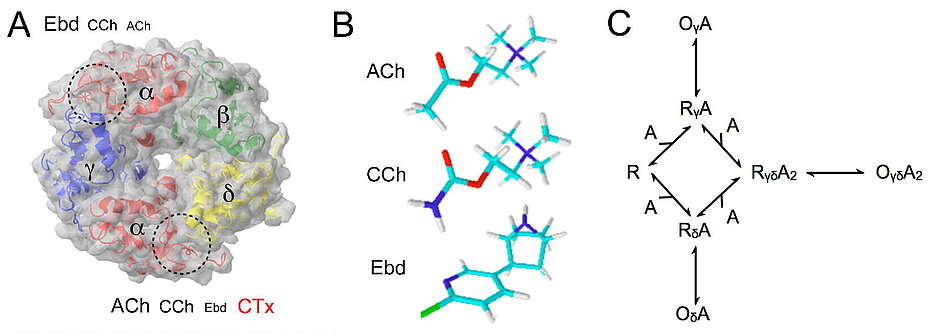
"Embryonic" muscle type nicotinic acetylcholine receptor-channels (nAChRs) bind ligands at interfaces of α- and of γ- or δ-subunits. αγ- and αδ-sites differ in affinity but their contributions in opening the channel remained elusive. We compared high resolution patch-clamp currents evoked by epibatidine (Ebd), carbamylcholine (CCh) and acetylcholine (ACh). Ebd binds with 75-fold higher affinity at αγ- than at αδ-sites whereas CCh and ACh prefer αδ-sites. Similar short (τO1), intermediate (τO2) and long types of openings (τO3) were observed with all three agonists. τO2 openings were maximally prevalent at low Ebd concentrations, binding at αγ-sites. Vice versa, τO1 openings appear to be generated at αδ-sites. In addition two types of bursts appeared: Short bursts of on average 0.75 ms (τB1) that should arise from the αγ-site, and long bursts of 12 to 25 ms (τB2) durations arising from double liganded receptors. Limited by the temporal resolution, the closings within bursts were invariant at 3 μs. Corrected for missed closings, in case of ACh the openings within long bursts lasted 170 μs, those in short bursts about 30 μs. Blocking αδ-sites with α-conotoxin M1 (CTx) eliminated both τO1 and τB2 and left only τO2 and the short τB1 bursts, as expected. Furthermore we found desensitization when the receptors bind ACh only at the αγ-site. When CTx was applied to `embryonic' mouse endplates, monoquantal current rise times were increased, and amplitude and decay time constants were reduced, as expected. Thus the αγ- and αδ-site of nAChRs elicit specific channel opening patterns.